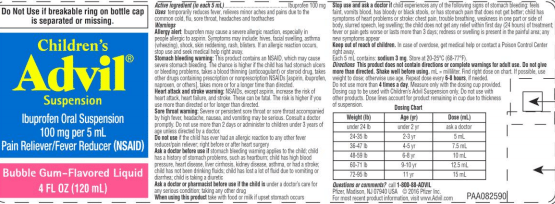
Pfizer has issued a voluntary recall for some Children’s Advil due to concerns of inaccurate dosage labeling that may result in overdoses. The company says that the dosage cup provided with the bottles is marked in teaspoons when the instructions are labeled in milliliters (mL). The recall affects the company’s Children’s Advil Suspension Bubble Gum Flavored product in four-ounce bottles.
Pfizer says that the concern of potential overdoses is possible due to the inaccurate labeling on the dosage cup. The company says that symptoms associated with an ibuprofen overdose include nausea, vomiting, headache, drowsiness, blurred vision and dizziness.
Children’s Advil is marketed as a temporary fever reducer that also relieves minor aches and pains due to the common cold, flu, sore throat, headaches and toothaches. The product was distributed nationwide to wholesalers, distributors and retailers in the United States from May 2018 through June 2018, according to Pfizer.
Packages containing the Lot number R51129, an expiration date of 11/20 and the UPC code of 3-0573-0207-30-0 are affected by this recall.
Consumers with questions about the recall can contact Pfizer Consumer Healthcare at 1-800-882-3845. Any adverse reactions to taking this product should be reported to a doctor and to the FDA MedWatch Adverse Event Reporting Program.


